


Maher Jabado
Review of PH Table and PPM Explanation
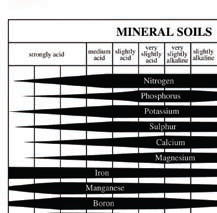
1) PH table :
This chart shows the relative availabilities
of most plant nutrients at various pH levels.
The width of each horizontal bar indicates
maximum availability at the widest point
and diminishing availability as the
bar narrows.
Applying nutrients in the proper balance
is essential for proper plant nutrition,
but maintaining the pH of the soil
mix so that these nutrients are available
for uptake is just as crucial.
From these charts it can be seen that an
optimum pH in soil mixes is around 6.5 -
7.5, whereas the optimum
for soilless mixes falls in the 5.5 – 6.5
range.
 |
 |
 |
 |
||
2) PPM Explanation
Often in designing fertilization programs,
the term ppm appears. Simply, this means
Part Per Million. One ppm of a certain nutrient,
such as nitrogen, means that the solution
contains 1 part of nitrogen in 1,000,000
parts of water by weight. There is an easy
formula which can be used to determine the
ppm of a solution: [(grams of fertilizer
product) / liter of water] x grade of fertilizer
x 10 = ppm
It works like this:
Start with Fetilizer Maher type 20-20-20
that contains 20% N.
-Objective 1: To find the ppm of nitrogen in a solution containing 1g of Maher type 20-20-20 in
100 litres of water, the equation is:
[1 (g of product) / liters of water in solution]
x 20 (grade of fertilizer) x 10 = 2 ppm
That means One gram of Maher 20-20-20 in
100 litres of water has 2 parts per million
of nitrogen in the solution.
-NEXT is Objective 2: To determine the number of grams required
to make up a 200 ppm solution of nitrogen
with Maher 20-20-20 fertilizer in 100 litres
of solution, simply divide 200 by 2. Your
answer is 100 g. litres of water in solution
100 (litres of water)